NON-INVASIVE ELECTROMAGNETIC THERAPIES (Part 1)
A report by: Jessica Connell-Glynn / Edited by the NYCRA-NEWS Editorial team
In 2015, Dr. Oz aired a program about an "invisible treatment" for chronic pain designed to "change the practice of medicine"- casting a wave of commercial attention to the patient community desperate for pain relief- and hope for the reversal of pain-related ailments. Pulsed Elecromagnetic Field Thereapy or PEMF is the induction of electromagnetic fields to promote the synthesis of cellular, muscular and skeletal matrix [1].
Electromagnetic fields (EMFs) provide a non-invasive, safe, and easy method to treat and rehabilitate a growing list of health issues. The therapeutic theory behind this innovation is designed to affect the body at the extracellular level- imposing the ability to transduce or separate mechanical charge to electrical energy. This has been reported to affect the alignment and physical properties of tissues & cell nutrition toward the healing process.[2] Electromagnetic fields (EMFs) and magnetic therapy presents a major increase in research attention in the past decade for its ability to directly treat the site of injury. [3]
Medical historians and publishers often attribute the scientific roots of electrical transduction therapy to NIKOLA TESLA (1856-1943) for his pioneer work in advanced electromagnetic field frequency. They align the success of non-invasive treatment innovations including the Pulsed Elecromagnetic Field Thereapy (PEMF) and the Transcranial Magnetic stimulation (TMS) to the philosophy of implementing electgromagnetic fields to stimulate cells. It is this same paradigm in patient care technology that enforces the very backbone of holistic and non-surgical ("no more scalpel") movement.
Conventional uses of magnetic therapy is widely found in the integrative and alternative (or wellness) communities. Some may recognize this science under other names including:
Wave Therapy Biofeedback Bio-resonance
Magnetic Therapy Electrostimulation Microcurrent Therapy
These devices vary in size & designs but these protocols are similar in application. The science employs a magnetic field for rehabilitation to treat and/or prevent diseases.
CLEARED FOR USES
In 1979, The Food and Drug Administration 1979 approved PEMF Therapy for the healing of non-union fractures [4]. Electrical stimulation of the spine (as part of spinal fusion procedures) for failed fusions and congenital pseudarthroses. In October 2008 the Food and Drug Administration approved the use of PEMF therapy for treatment of major depressive disorder in PD patients who failed to achieve satisfactory improvement from very high dosages of antidepressant medications [5].
Clinical research has also been highly dedicated towards mental health. In 2006, the FDA approved PEMF Therapy for treatment of depression and anxiety. [7-9] Further reports have presented an est. 30% of depression cases have a resistance to antidepressant drugs, where Repetitive Transcranial Magnetic Stimulation (rTMS) and the application of Transcranially applied Pulsed Electromagnetic Fields (T-PEMF) has shown positive results in combination with antidepressants in patients with treatment-resistant depression[10].
In 2019 the wearable RecoveryRx PEMF Device was FDA Cleared for "Adjunctive treatment of postoperative pain". This technology achived market clearance for its drug-free (non-opiod) PostOp effects. Andrew Whelan, President, expressed confidence in seizing the market opportunity: “We are delighted that the FDA has recognized the potential of RecoveryRx in transforming postoperative pain care. RecoveryRx, with its high degree of safety, excellent clinical evidence of effectiveness and exceptional cost-benefit, will become the standard of care.” [11-12]
PEMF: HISTORY & EXPANSION OF MODERN TARGET ISSUES
Claims of electricity induction in aiding bone repair has been reported as far back as the mid 1800's, but it wasn't until the mid-1950's to the '70's that the scientific community significantly advanced its research toward the treatment of delated fractures in Western Europe and the US. Recent reviews have shown benefits of PEMF to include: neurological disorders, vascular disease, lung disorders, GI (gastrointenstinal) disease, rheumatic disease, dermatological issues, inflammation, tumors and immunological issues for both humans and animals.[14] Clinical research studies have reported benefits for a host of specific issues including: musculoskeletal(MSK) Pain [15], bacteria and parasites, OstheoArthritis, mental health disorders (Depression), Multiple Sclerosis[16-17], Cancer[18] and Alzheimer's Disease [19].
TMS and PEMF USE IN PARKINSON'S DISEASE
Parkinson’s disease (PD) is one of the most common progressive nervous system disorders that affects movement. It is a neurodegenerative disorder second only to Alzheimer’s disease, accompanied by the impairment of the cortico-subcortical excitation and inhibition systems, hence belonging to the involuntary movement diseases. In the first PD patients treated with high-frequency TMS in 1993, motor symptoms, tremor, rigidity and akinesia improved significantly allowing to decrease the administration of l-dopa by a mean of 55% [20].
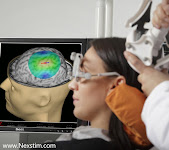
TMS FOR DEPRESSION: Transcranial Magnetic Stimulation October 4, 2021- TMS, or transcranial magnetic stimulation, is the use of magnets external to the body to activate tissue inside the body (so you're not having to open the patient up). Based on Faraday’s Law, a magnetic field produced outside of a patient’s head can permeate non-invasively through a patient’s head and induce an electric field that has the capacity to activate neurons in the brain. We induce current at a distance inside the brain and cause the neurons to fire where we induce that current. This means that where we depolarize, we cause the brains neurons to fire. TMS artificially stimulates the brain and causes the neurons to fire. Navigation technology allows us to see precisely where in the brain where we are stimulating. (see complete article) |
The AOPP: Explaining How PEMF Works
9/25/2021: An exploratory review* of the PEMF science is under discussion between Dr. Robert Bard and THE AOPP FOUNDATION (Association of PEMF Professionals) which aims to provide clarity on the science behind its 'invisible healing' concept. AOPP aligned with the Integrative Pain Healers Alliance (Dr. Bard's wellness network) and saw the need for public clarity in how PEMF works, what actual uses they are cleared for as well as the importance of public guidance on the market.
Dr. Jerry Dreesen, President of the AOPP and certified chiropractor by trade is an avid user of this therapeutic technology for his patients on a variety of disorders. "So PEMF did start out on the animal side... it was originally used for seed germination -and moved over to the animal side. When they eventually started working with humans, evidence of creating better blood flow as part of the body's detoxification. We're also finding that with PEMF is that the body is utilizing medication even better - so it's absorbing it and using it more versus some people can be getting a reaction to better absorption of their medications."
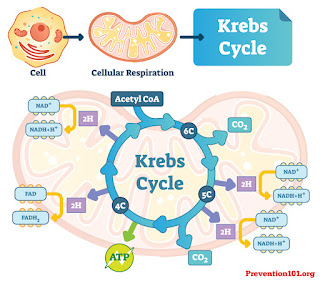
Dr. Dreesen further explains the physiological and biological reactions to electromagnetic science and its research - going as far back as the Krebs cycle (otherwise known as The citric acid cycle (CAC) – or the TCA cycle (tricarboxylic acid cycle)- which we all had to learn. During that process. one of the by products is nitric oxide and then the other one is of course, taking ADP and attaching the phosphate to it and making ATP-- creating the "fuel" for the body. PEMF sparking this process is great especially for sports where it induces renewed energy to fatigued muscles. For the same reasons why it works really well on animals, the same goes for humans after a workout as it quickly revitalizes the target area by increasing absorption of oxygen and energy levels.
Dr. Dreesen shares the rehabilitative theories and applications for MSK injuries- including sprains, strains, joint issues, arthritis pain and other chronic inflammation. He describes its performance on a cellular level and controlling inflammation versus "just telling the brain that there's no pain there at all... it's not masking pain, it's physically going in and eliminating the creation of inflammation. As a by-product of going in and oxygenating tissue, creating stem cells, eliminating causing the cellular walls to get rid of product and bringing in nutrients, it rebuilds and refreshes the joints." He furthers, "for people who have had past injuries in their joints and for whatever reason, never healed properly, PEMF can offer significant change- not only in reducing inflammation, but potentially preventing it from coming back!"
MEETING DEVELOPERS
Tech critic and test-driver Dr. Robert Bard met with a short list of AOPP members including Patrick Ziemer, development facilitator and CTO of Aura Wellness PEMF (Aurawell.com) to discuss the growing number of health issues and disorders that neuromagnetic science is slated to manage, treat and even resolve. As part of his global crusade to support non-invasive medicine, Dr. Bard recognizes the advancing benefits of PEMF throughout the wellness community and is evaluating all devices including the AuraPulse™ technology.
Mr. Ziemer is a consumer advocate for the AOPP and publishes educational posts about the benefits and his evidence of the PEMF technology to align with his critical thoughts on the industry. From our interview, he shares his expansive research on the science, allotting for the limited number of actual models that are FDA cleared, approved or registered. As part of his advocacy, Mr. Ziemer describes the confusion about neuromagnetic therapy to come from companies who presents “rogue claims” about their specific devices. Many lower-end companies tend to lack the proper extensive safety testing of their devices and espouse claims “from studies done by others that are FDA approved. For example, companies will make claims that their devices will heal broken bones. "PEMF has been known to do this, but we can't legally say that about OUR specific device without doing the studies (ourselves) from a formal FDA 510K clearance process … and all companies should take the same responsibility about their work”.
Mr. Ziemer’s history with PEMF started around 2013, co-developing and promoting the therapeutic use high-voltage PEMF for the equestrian society. His device appealed greatly to the veterinary community for its ability to address the many health concerns of our four legged friends who rarely complain. Therapeutically, his PEMF has shown to improve horses’ circulation, increasing blood oxygen, lymphatic stimulation, relaxing muscle spasms, relieving tension, enhancing muscle tone, and increasing the range of motion in high performance horses. These same health issues expanded his popularity to treat a growing range of patient breeds including cattle, livestock and dogs across the country.
In 2020, Mr. Ziemer’s PEMF officially made its way to human patients at the launch of Aura Wellness- carrying a professional grade, high voltage PEMF device. Applying much of his field expertise with animals, Mr. Ziemer achieved tremendous R&D advantage to apply to his human prototype. His AuraPulse™ model is completely programmable with adjustable intensity levels, digital timers, plug-and-play applicators, and customized session programs. Dr. Bard is reviewing* all PEMF devices like the AuraPulse™ for its effects and claims for improved wellness.
"Our first test subject was 4 legged and suffered from arthritis. While on the magnetic coil, the poodle visibly became calmer, eventually going to sleep inside the treatment ring. The dog’s handler saw the improvement in the gait and asked to have her shoulder treated. After a 10 minute session, our 2 legged patient reported mild improvement on a chronic rotator cuff disorder and great improvement 12 hours later."
*It is to be noted that beta testing and clinical evaluation reviews of all medical devices are restricted to their intended uses according to their designated FDA clearances.
CONTRIBUTORS
JESSICA CONNELL-GLYNN, LCSW, CPC, CEC is a columnist for Prevention101 newsletter. She often reports on topics concerning mental health, neurological disorders and medical innovations that pertain to 'modern wellness'. As a therapist and mental health coach, Jessica's primary work involves providing direct support in managing symptoms of personal anxiety and panic. Jessica is also currently leading an interdisciplinary research project with a team of mental health and professionals in neurology to review the current care standards and collaborate on new protocols that target, assess and diagnose psychological disorders.(https://cmpcnyc.com/)
Dr. JERRY DREESSEN is both Trauma Team Qualified and Hospital Qualified treating chiropractor in Mountlake Terrace, WA. He is the Executive Director for the Association of PEMF Professionals- the largest professional PEMF organization in the United States. AOPP attracts the most principled and accomplished PEMF user- setting standards by providing certification for all PEMF users, as well as Continuing Education (CEU's) for all members to maintain best practices and techniques. (www.backtoaction.com)
SPECIAL THANKS
The editors of this spotlight proudly gives thanks to Ms. Suzanne Wheeler for her generosity in sharing her story and her resources with us. Additional thanks to Dr. Jerry Dreessen of the AOPP (Association of PEMF Professionals) and Pat Ziemer of Magnawave Inc. and Aura Wellness PEMF for coordinating our interviews, shared countless materials and conducted unending support to help our educational program bring new light to PEMF technology for chronic disorders and supportive testimonials in alternative therapeutics.
The editors of this spotlight proudly gives thanks to Ms. Suzanne Wheeler for her generosity in sharing her story and her resources with us. Additional thanks to Dr. Jerry Dreessen of the AOPP (Association of PEMF Professionals) and Pat Ziemer of Magnawave Inc. and Aura Wellness PEMF for coordinating our interviews, shared countless materials and conducted unending support to help our educational program bring new light to PEMF technology for chronic disorders and supportive testimonials in alternative therapeutics.
VIEWPOINTS from the Healthcare Community It is refreshing to learn about other forward thinking modalities and innovations in the healthcare field. These Innovations like pulsed electromagnetic field therapy(PEMF) and transcranial magnetic stimulation (TMS) are technologies that utilize non-invasive electromagnetic stimulation of cellular metabolic processes within various places in our body. The endgame of the technologies is for diagnostic information and therapeutic intervention of systemic disease. PEMF and TMS have proven that electromagnetic fields can provide non-invasive methods to diagnose and treat health concerns such as depression, pain, MS, Cancer, Alzheimer’s Disease, Parkinson’s Disease, healing responses, and Overall cardiovascular anomalies. This is so exciting because we can use this technology two recognize early metabolic and possibly genetic changes that could lead to accelerated aging disease and death. Also, we can use this technology as an intervention to the changes and correct the path of health. Both of these technologies have shown efficacy ( FDA approval) and promise ( more research is being done). There is no question that LESS IS MORE! That is if we recognize pathological conditions early and can correct them with little or no pharmacological or surgical intervention; “everybody wins!” - By Randall Weisel DDS,MPS (www.laserhygienics.com) |
REFERENCES
1) Ehnert, Sabrina; Schröter, Steffen; Aspera-Werz, Romina H.; Eisler, Wiebke; Falldorf, Karsten; Ronniger, Michael; Nussler, Andreas K. (December 2019). "Translational Insights into Extremely Low Frequency Pulsed Electromagnetic Fields (ELF-PEMFs) for Bone Regeneration after Trauma and Orthopedic Surgery". Journal of Clinical Medicine. 8 (12): 2028. doi:10.3390/jcm8122028. PMC 6947624. PMID 31756999.
2) ACCELERATION OF FRACTURE REPAIR BY ELECTROMAGNETIC FIELDS. A SURGICALLY NONINVASIVE METHOD: First published: October 1974 https://doi.org/10.1111/j.1749-6632.1974.tb26794.x
3) Electromagnetic Field Therapy: A Rehabilitative Perspective in the Management of Musculoskeletal Pain – A Systematic Review J Pain Res. 2020; 13: 1385–1400. Published online 2020 Jun 12. doi: 10.2147/JPR.S231778 PMCID: PMC7297361 PMID: 32606905 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7297361/
4) Electrical stimulation of the spine as an adjunct to spinal fusion procedures". Blue Cross & Blue Shield of Mississippi. Archived from the original on 2015-04-02. Pulsed electromagnetic field systems with FDA PMA include the EBI Bone Healing System from Electrobiology, Inc., which was first approved in 1979 and indicated for nonunions, failed fusions, and congenital pseudarthroses; and the Cervical-Stim from Orthofix, which was approved in 2004 as an adjunct to cervical fusion surgery in patients at high risk for non-fusion. https://web.archive.org/web/20150402122818/http://www.bcbsms.com/index.php/index.php?id=200&action=viewPolicy&path=%2Fpolicy%2Femed%2FElectrical+Stimulation+of+the+Spine+as+an+Adjunct+to+Spinal+Fusion+Procedures.html&source=emed
5) Mechanisms and therapeutic applications of electromagnetic therapy in Parkinson’s disease (BMC/SPRINGER) Behavioral and Brain Functions-
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4562205/#CR125
6) Clinical significance of transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant depression: synthesis of recent data.
Demitrack MA, Thase ME Psychopharmacol Bull. 2009; 42(2):5-38. https://pubmed.ncbi.nlm.nih.gov/19629020/
7) Pulsed Electro Magnetic Fields (PEMF) in Depression (PEMF) https://clinicaltrials.gov/ct2/show/NCT03556735
8) Demitrack MA, Thase ME. Clinical significance of transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant depression: synthesis of recent data. Psychopharmacol Bull. 2009;42(2):5–38 - https://pubmed.ncbi.nlm.nih.gov/19629020/
9) Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center- https://pubmed.ncbi.nlm.nih.gov/22579164/
10) Biological Psychiatry (Journal) /ARCHIVAL REPORT| VOLUME 68, ISSUE 2, P163-169, JULY 15, 2010 Transcranial Low Voltage Pulsed Electromagnetic Fields in Patients with Treatment-Resistant Depression https://www.biologicalpsychiatryjournal.com/article/S0006-3223(10)00162-9/fulltext
11) US FDA- https://www.accessdata.fda.gov/cdrh_docs/pdf19/K190251.pdf
12) Press Release/BioElectronics Announces FDA Market Clearance for Its Non-Opioid Postoperative Pain Therapy https://www.globenewswire.com/en/news-release/2019/07/01/1876675/0/en/BioElectronics-Announces-FDA-Market-Clearance-for-Its-Non-Opioid-Postoperative-Pain-Therapy.html
13) 12/10/2009 -FDA clears Nexstim´s Navigated Brain Stimulation for non-invasive cortical mapping prior to neurosurgery (Nexstim Press release)
https://nexstim.com/news-and-events/news/press-release/news/fda-clears-nexstims-navigated-brain-stimulation-for-non-invasive-cortical-mapping-prior-to-neurosur/
14) Jerabeck, J; Pawluk, W (1998). Magnetic therapy in eastern Europe : a review of 30 years of research. W. Pawluk. ISBN 0-9664227-0-8.
15) Thomas AW, Graham K, Prato FS, McKay J, Forster PM, Moulin DE, et al. A randomized, double-blind, placebo-controlled clinical trial using a low-frequency magnetic field in the treatment of musculoskeletal chronic pain. Pain Res Manage J Can Pain Soc (journal de la societe canadienne pour le traitement de la douleur) 2007;12(4):249–258 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2670735/
16) Lappin MS, Lawrie FW, Richards TL, Kramer ED. Effects of a pulsed electromagnetic therapy on multiple sclerosis fatigue and quality of life: a double-blind, placebo controlled trial. Altern Ther Health Med. 2003;9(4):38–48. https://pubmed.ncbi.nlm.nih.gov/12868251/
17) Richards TL, Lappin MS, Acosta-Urquidi J, Kraft GH, Heide AC, Lawrie FW, et al. Double-blind study of pulsing magnetic field effects on multiple sclerosis. J Altern Complement Med. 1997;3(1):21–29. doi: 10.1089/acm.1997.3.21. https://pubmed.ncbi.nlm.nih.gov/9395691/
18) Barbault A, Costa FP, Bottger B, Munden RF, Bomholt F, Kuster N, et al. Amplitude-modulated electromagnetic fields for the treatment of cancer: discovery of tumor-specific frequencies and assessment of a novel therapeutic approach. J Exp Clin Cancer Res. 2009;28:51. doi: 10.1186/1756-9966-28-51
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2672058/
19) Arendash GW, Sanchez-Ramos J, Mori T, Mamcarz M, Lin X, Runfeldt M, et al. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer’s disease mice. J Alzheimers Dis. 2010;19(1):191–210. https://pubmed.ncbi.nlm.nih.gov/20061638/
20) Behav Brain Funct. 2015; 11: 26. Published online 2015 Sep 7. doi: 10.1186/s12993-015-0070-z
PMCID: PMC4562205 PMID: 26347217 Mechanisms and therapeutic applications of electromagnetic therapy in Parkinson’s disease Maria Vadalà, Annamaria Vallelunga, Lucia Palmieri, Beniamino Palmieri, Julio Cesar Morales-Medina, and Tommaso Iannitti corresponding author https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4562205/
21) The Krebs Cycle: https://en.wikipedia.org/wiki/Citric_acid_cycle
PHOTO CREDITS
* Nikola Tesla in or around 1890, when the inventor was in his mid-30s. | Photo is in the public domain. Image courtesy of the Library of Congress. https://www.energy.gov/articles/top-11-things-you-didnt-know-about-nikola-tesla
Disclaimer & Copyright Notice: The materials provided on this website/web-based article are copyrighted 2021 and the intellectual property of the publishers/producers (The NY Cancer Resource Alliance/IntermediaWorx inc. and Bard Diagnostic Research & Educational Programs). It is provided publicly strictly for informational purposes within non-commercial use and not for purposes of resale, distribution, public display or performance. Unless otherwise indicated on this web based page, sharing, re-posting, re-publishing of this work is strictly prohibited without due permission from the publishers. Also, certain content may be licensed from third-parties. The licenses for some of this Content may contain additional terms. When such Content licenses contain additional terms, we will make these terms available to you on those pages (which his incorporated herein by reference).The publishers/producers of this site and its contents such as videos, graphics, text, and other materials published are not intended to be a substitute for professional medical advice, diagnosis, or treatment. For any questions you may have regarding a medical condition, please always seek the advice of your physician or a qualified health provider. Do not postpone or disregard any professional medical advice over something you may have seen or read on this website. If you think you may have a medical emergency, call your doctor or 9-1-1 immediately. This website does not support, endorse or recommend any specific products, tests, physicians, procedures, treatment opinions or other information that may be mentioned on this site. Referencing any content or information seen or published in this website or shared by other visitors of this website is solely at your own risk. The publishers/producers of this Internet web site reserves the right, at its sole discretion, to modify, disable access to, or discontinue, temporarily or permanently, all or any part of this Internet web site or any information contained thereon without liability or notice to you.