Written by: Dr. Robert L. Bard
From a 2019 study (by Raizman et al) about use of this technology (MolecuLight i:X) with debridement, scans of 22 wounds were conducted under standard and violet light in patients after debridement. Scans, performed non-invasively at the point-of-care, demonstrated remaining bacteria/biofilm signal in 100% of wounds after sharp debridement, triggering additional removal of tissue. [1]
Re-scanning demonstrated a marked decrease or complete removal of bacteria in most wounds, yet a subset showed persistent or increased bacterial signals post debridement. The authors, aided by the knowledge gained from this technology, proposed “the subgroup with persistent bacterial fluorescence post-debridement was at increased risk of deep compartment infection and required more frequent debridement and/or antibiotics.” [1]. See example of this in figure 2 (tissue in green, bacteria in red). Others have demonstrated ability to markedly decrease antibiotic usage using this technology, showing great potential for stewardship efforts [2].
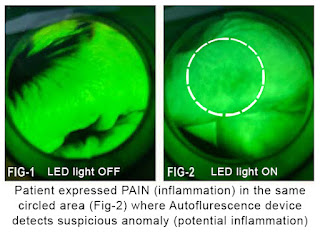
Currently, the technology can take direct images of the skin or mucosal surface to indicate bacteria on the tongue or oral lining. Exploratory reviews of this imaging device also shows possible benefits in scanning potential biopsy material with violet light at the tissue and see if it's suggestive for cancer or inflammatory disease.Autofluorescence has been proven and used worldwide to show inflammatory changes and bacterial infection. It's used to find both Gram‐negative bacteria and Gram‐positive bacteria, aerobic and anaerobic. (Fig‐1) The illustrated scan shows the normal skinfolds as green and the skin image is homogeneous (there's nothing dark). (Fig‐2) The scan of the finger on the opposite hand which the patient expressed chronic irritation. Upon initial observation, it appears that Autofluorescence may detect inflammation through the upper dermis, a change documented visually and by ultrasound imaging as minimal epidermal thickening associated with inflammatory skin disease (mild type). When we turned on the fluorescence scan, the exact one centimeter area of redness on the skin corresponded to the one centimeter darkened area on the finger.
SCAN 2: CLINICAL IMAGING & OBJECTIVE DATA MEASURING WITH ULTRASOUND
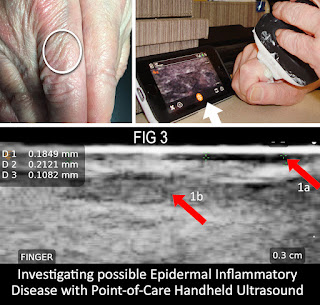
(Fig-3) For my diagnostic research projects in dermal lesions and infections, one of my "weapons of choice" is the doppler ultrasound. From tumors to traumas, radiologists and clinicians rely on feature-rich computerized systems that provided remarkable patient data for its non‐invasive and real-time view of inflammatory disease to align with our study of the Autofluorescence for its work on atopic dermatitis and other viral diseases (ie. Covid-19) which may affect the skin and be sampled with this noninvasive technology. Since it hasn't been clinically documented in the dermal tissues, this is an opportunity to form a new type of imaging that is non-invasive to consider avoiding biopsies on children.In the case of Fig-3, I used a high-frequency setting on a popular handheld point of care ultrasound probe to recognize a 0.3cm depth scan of the same area of the finger that was previously scanned by the fluorescence device. The top right arrow (1a) shows the enlarging dark stripe indicating inflammatory thickening or the epidermis. The middle grey area (arrow 1b) represents subdermal inflammation of subcutaneous tissue or mild inflammatory skin disease - confirming the prior evaluation.
EXPANSION POTENTIAL: RESEARCH
Fluorescence imaging is proven to have a four‐millimeter depth of diagnostic accuracy in the tongue or mucous membranes‐ similar to the cervix or the vagina or the intestine lining. There is a rich literature of its use in skin wounds and wound infection. However, it's use in skin diseases has not been fully explored‐ so this is a beta test to see if and how it will work in non‐invasive diagnosis of inflammatory skin disease, especially in children. Exploring this technology is of great importance because dermatitis is recognized as a major pediatric disorder. Also disabling inflammatory chronic skin disease (psoriasis, rosacea) are a major focus of successful treatment by pharmacology companies.
by: Randall Weisel, DDS (Excerpt from Journal of Dentistry and Oral Sciences/ "Auto Fluorescence Allows Us to Detect Early Signs of Oral Cancer and Much More")
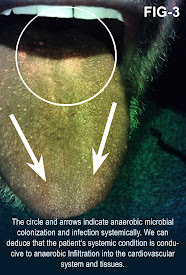
Review of autofluorescence enables us to objectively examine the oral cavity for inflammation and infection. Many systemic diseases are perpetuated by microorganisms that colonize in the oral environment. They enter the cardiovascular system by enzymatic processes that open the oral mucosa to allow their entry. A majority of the microbes are anaerobes and/or facultative anaerobes. When they enter the host, they metabolize blood. Their waste by products contains iron elements within a compound called porphyrin. Porphyrin will fluoresce when exposed to certain wavelengths of light. Healthcare providers can utilize this natural occurring process to objectively see these harmful pathogens. This may indicate that the host has a Sleep Related Breathing Disorder (SRBD). Sleep apnea is a primary disorder of SRBD’s. This technology offers medical and dental fields a screening tool for a pandemic healthcare problem.
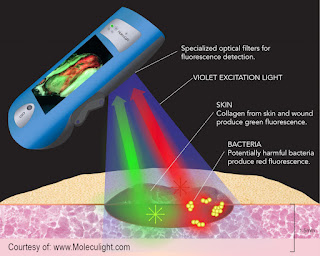
Pathogenic microbial presences and the size of the colony (the bioload) can be relatively determined [19]. Fluorescence visualization of red-orange means bacterial pathogens are present. Presence of bluish green/bright white indicates presence of Pseudomonas [19]. The conditions for these pathogens to exist can occur within individuals with Sleep Related Breathing Disorders (SRBD). Sleep Apnea is the most prevalent of these disorders [20]. Researchers should focus their attention to the dorsum of the tongue ecosystem. Autofluorescence, performed with technology that utilizes electromagnetic spectral ranges and special filters that are components of the Velscope, allows visualization of pathogenic microbes. Scientific research demonstrates that oral pathogens (Pg, Aa, and Fn) may be direct and/or indirect causative agents of systemic diseases. These oral pathogens cause inflammation and infection.
SPECIAL THANKS to Dr. Monique Rennie, Director of Scientific Affairs and Global Engagement at Moleculight for her generous technical support and assistance greatly added to the quality and integrity of this feature article.
REFERENCES:
1) Raizman R et al. Use of a bacterial fluorescence imaging device: wound measurement, bacterial detection and targeted debridement. J Wound Care (2019). https://pubmed.ncbi.nlm.nih.gov/31825778/
2) Price N. Routine fluorescence imaging to detect wound bacteria reduces antibiotic use and antimicrobial dressing expenditure while improving healing rates: retrospective analysis of 229 foot ulcers. Diagnostics 2020;10:927.
19) Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Advances in Wound Care. 2021. https://pubmed.ncbi.nlm.nih.gov/32870774/
20) Sleep-Disordered Breathing.chapter 23.Thoracic.org. 237-247. Boillot A, Demmer RT, Mallat Z, Sacco RL, Jacobs DR, Benessiano J, et al. Periodontal microbiota and phospholipases: the oral infections and vascular disease epidemiology study (INVEST). Atherosclerosis. 2015;242(2):418-23. PubMed | CrossRef
21) Bale BF, Doneen AL, Vigerust DJ. High-risk periodontal pathogens contribute to the pathogenesis of atherosclerosis. Postgrad Med J. 2017;93(1098):215-20. PubMed | CrossRef
22. Boillot A, Demmer RT, Mallat Z, Sacco RL, Jacobs DR, Benessiano J, et al. Periodontal microbiota and phospholipases: the oral infections and vascular disease epidemiology study (INVEST). Atherosclerosis. 2015;242(2):418-23. PubMed | CrossRef
23) Desvarieux M, Demmer RT, Jacobs Jr DR, Rundek T, Boden-Albala B, Sacco RL, et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens. 2010;28(7):1413. PubMed | CrossRef